
Many UC-authored scholarly publications are freely available on this site because of the UC's open access policies.
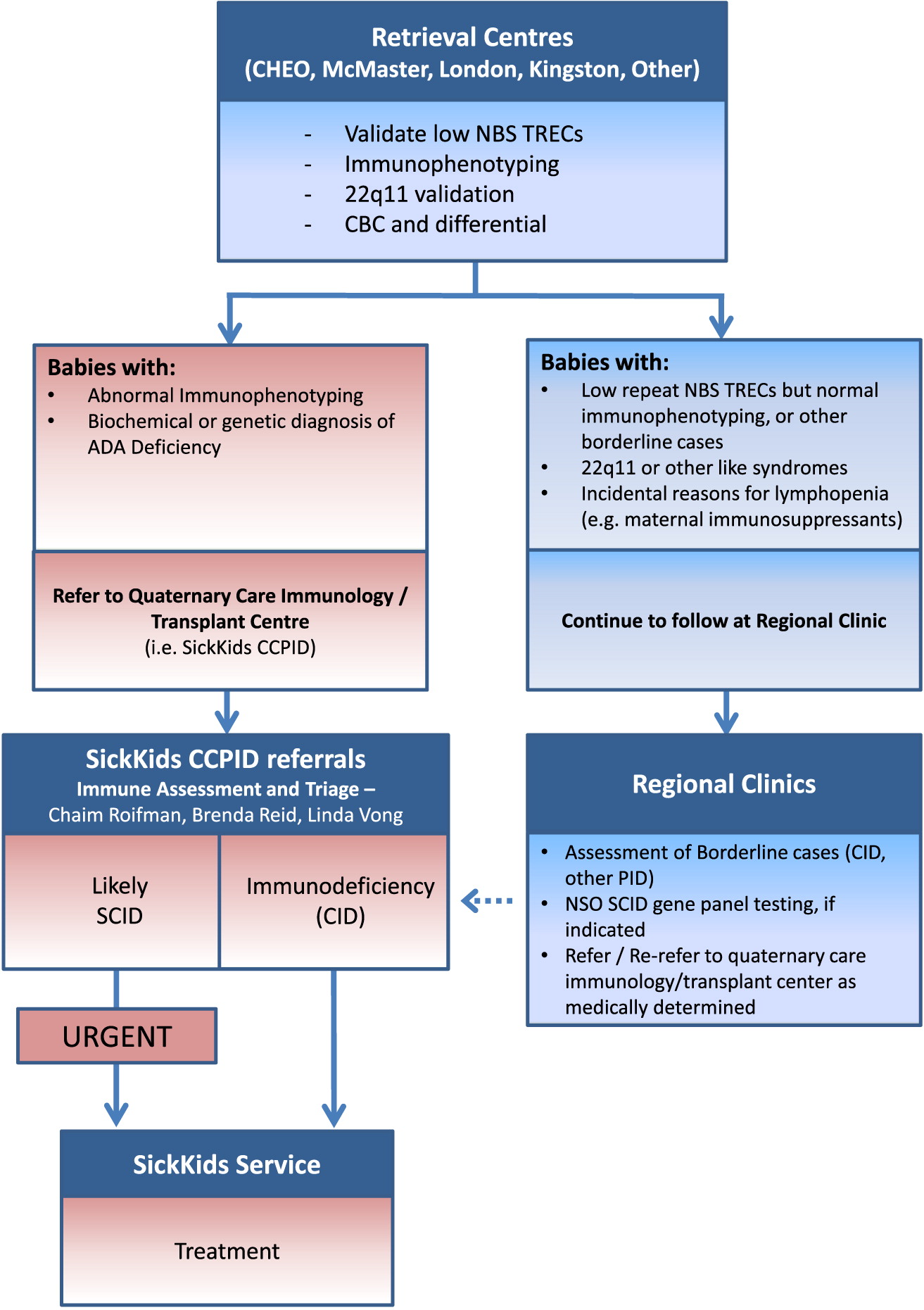
The usefulness of detection of non-SCID T-cell lymphopenias by the same screening remains to be determined. Conclusions and relevanceNewborn screening in 11 programs in the United States identified SCID in 1 in 58,000 infants, with high survival. Variations in definitions and follow-up practices influenced the rates of detection of non-SCID T-cell lymphopenia.

'Many of these conditions are very uncommon or rare,' says Friesen. Manitoba Health Minister Cameron Friesen says newborn screening looks for easily detectable and preventable diseases linked to inherited and non-heritable disorders. Additional interventions for SCID and non-SCID T-cell lymphopenia included immunoglobulin infusions, preventive antibiotics, and avoidance of live vaccines. SCID is a group of rare genetic diseases affecting a childs immune system. Survival of SCID-affected infants through their diagnosis and immune reconstitution was 87% (45/52), 92% (45/49) for infants who received transplantation, enzyme replacement, and/or gene therapy. ResultsScreening detected 52 cases of typical SCID, leaky SCID, and Omenn syndrome, affecting 1 in 58,000 infants (95% CI, 1/46,000-1/80,000). Newborns with SCID appear healthy, but remain extremely vulnerable to. SCID is a primary immune deficiency that is characterized by the lack of a functioning immune system. Incidence and, where possible, etiologies were determined. Severe Combined Immunodeficiency (SCID) Newborn Screening was added to the Recommended Uniform Screening Panel (RUSP) in May of 2010 following rigorous state based implementation programs in Massachusetts and Wisconsin. Main outcomes and measuresInfants with SCID and other diagnoses of T-cell lymphopenia were classified.
#SCID NEWBORN SCREENING PLUS#
Representatives from 10 states plus the Navajo Area Indian Health Service contributed data from 3,030,083 newborns screened with a TREC test. Infants born from the start of each participating program from January 2008 through the most recent evaluable date prior to July 2013 were included. SettingRepresentatives in states conducting SCID newborn screening were invited to submit their SCID screening algorithms, test performance data, and deidentified clinical and laboratory information regarding infants screened and cases with nonnormal results. DesignEpidemiological and retrospective observational study. ObjectivesTo present data from a spectrum of SCID newborn screening programs, establish population-based incidence for SCID and other conditions with T-cell lymphopenia, and document early institution of effective treatments. The incidence of SCID is estimated at 1 in 100,000 births. Currently 23 states, the District of Columbia, and the Navajo Nation conduct population-wide newborn screening for SCID. ImportanceNewborn screening for severe combined immunodeficiency (SCID) using assays to detect T-cell receptor excision circles (TRECs) began in Wisconsin in 2008, and SCID was added to the national recommended uniform panel for newborn screened disorders in 2010.


 0 kommentar(er)
0 kommentar(er)
